genetic “selection experiment” that has been underway since the origin of the vertebrate limb, our lab studies the Lesser Egyptian Jerboa, Jaculus jaculus, as a new experimental system. The jerboa is closely related to the laboratory mouse, enabling direct comparison to an established model system with very similar genomic architecture and developmental staging, and yet with extremely divergent limb morphology. This desert-adapted bipedal rodent has normally proportioned forelimbs with five fingers and extraordinarily long hindlimbs with three toes on its disproportionately large feet, fused metatarsals, and an absence of foot muscles.
The goal of our research program is to capitalize on extreme divergence in the jerboa limb as a means to explore the broader and profoundly important relationship between gene regulatory landscapes and phenotypic malleability.
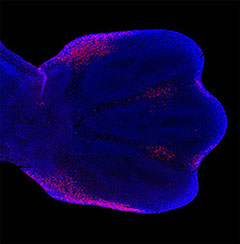
How are the correct numbers and
positions of the digits established?
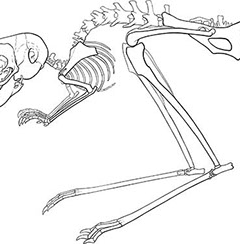
What mechanisms determine the size and relative
proportions of individual skeletal elements?
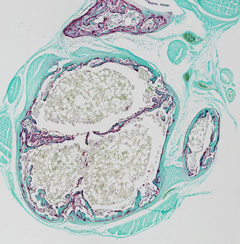
How does a bone fuse with its adjacent neighbor?
How is bone reshaping controlled?
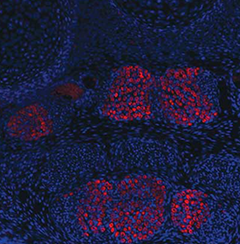
When a genetic alteration changes the morphology of a
tissue, how does that affect the development of
integrated tissue types?